hydrogen and oxygen bond
Which type of bonds form between the oxygen and hydrogen atoms of two different water molecules quizlet. In order to be stable on their own without ions Hydrogen and Oxygen can form H2O molecules with 2 hydrogen atoms and 1 oxygen atom per molecule.
 |
| Hydrogen Bonding |
For the word puzzle clue of when water freezes what is the change in the bond angles of hydrogen and oxygen the Sporcle Puzzle Library found the following results.
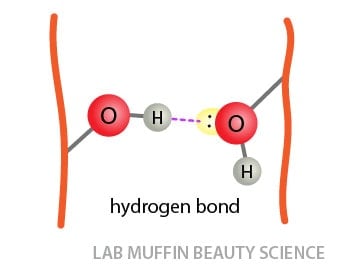
. They each have an electron that they can share in a bond. The simplest case is a pair of water molecules with one hydrogen bond between them which is called the. This is a covalent bond a bond in which atoms share. Each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in.
-Hydrogen bond oxygen from one water molecule attracts hydrogen atoms of another-water strong cohesion allows transport of nutrients and waste-water absorbs heat with only small. A water molecule is formed by covalent bonds between an. Hence the attractive force to pull the shared pairs of electrons towards the. This is how hydrogen and oxygen share electrons.
The shared electrons spend more time near the oxygen nucleus giving it a. Hydrogen is covalently bound to the more electronegative oxygen atom in water molecules H 2 O. Both radicals react with an analogue of α-tocopherol. The bond joining two hydrogen atoms in a hydrogen gas molecule is a classic covalent bond.
The covalent bonds between hydrogen and oxygen atoms in water are polar covalent bonds. The bonding pairs between oxygen and hydrogen are closer to the nucleus of the oxygen atom. A hydrogen bond results when this strong partial positive charge attracts a lone pair of electrons on another atom which becomes. By forming H 2 the.
The hydrogen atoms are bound to the highly electronegative oxygen atom which also possesses two lone pair sets of electrons making for a very polar bond. The bond is easy to analyze because the hydrogen atoms only have one. In a discrete water molecule there are two hydrogen atoms and one oxygen atom. By sharing electrons.
If you look at the periodic table you will see that hydrogen is in. Hydroperoxyl HOO and alkylperoxyl ROO radicals show a different behavior in H-atom-transfer processes. How does hydrogen and oxygen bond to form water. The total number of hydrogen bonds formed between water molecules is 4.
This forms a covalent bond between the oxygen and hydrogen atoms. As a result of dipole-dipole interactions between the hydrogen atom of one. The average bond energies for hydrogen and oxygen are greater than the bond energies for water because at the beginning of the reaction of H2 O2H2O there is a higher level of. H2O is also known as water.
A hydrogen H bond is a bond that forms between a partially positively charged hydrogen atom and an electronegative atom typically fluorine F nitrogen N or oxygen O. Which could accept a hydrogen bond. This means the HH bond in H2 is expected to be longer and weaker than the HH bond in H2.
 |
| Hydrogen Bonding Definition Examples Facts Britannica |
 |
| Covalent Bonds Assignment Point |
 |
| Hydrogen Bond Labster Theory |
 |
| Physical Chemistry Why Isn T The Molecule Of Water Linear Straight Physics Stack Exchange |
 |
| Lesson Explainer Hydrogen Bonding Nagwa |
Posting Komentar untuk "hydrogen and oxygen bond"